New editions of United States General Pharmacopeia Chapter <857> and European Pharmacopoeia Chapter 2.2.25, giving guidance on instrument qualification for ultraviolet and visible spectrophotometry, have recently been published. The former becomes mandatory on 1st December 2019 and the latter on 1st January 2020. Both introduce new approaches to qualification and suggest a variety of new reference materials for qualification measurements. While there are several areas of conformity between the two new standards, there are also some important differences.

What do they have in common?
Earlier versions of both standards contained a fairly limited set of tests to control an instrument’s performance for wavelength, absorbance, stray light and resolution (spectral bandwidth). Provided an instrument passed these tests it could be claimed that it was ‘pharmacopoeia compliant’. The limitation of this approach is that a qualification carried out under one set of operating conditions might not be valid under another set. A simple example would be a qualification carried out in the visible using one light source when the actual analysis was to be carried out in the UV region using a different source. While the same parameters must still be qualified, the requirement is now to demonstrate ‘fitness for purpose’, namely that the instrument has the performance to undertake the actual analysis to the required accuracy and precision. The user must therefore determine, for these four parameters, the range over which the system will be used in the actual analysis and prove compliance over that range. One consequence of this is that the simplistic approach often adopted in the past – one qualification test for each of the four parameters – may not suffice. Both standards now recommend, directly or by implication, that the values of the references used for qualification should ‘bracket’ the values to be used in the proposed analysis, which may mean that more than one reference is required. There is also a specific new requirement, in both standards, to qualify absorbance linearity: this will almost certainly require the use of more than one absorbance reference. To help meet these new requirements, additional reference materials are cited in both standards. Both standards also allow – the USP strongly advocates – the use of commercially available Certified Reference Materials.
How do they differ?
The major difference between the two new standards is their scope. Whereas USP <857> is limited to UV-visible spectrophotometers as described in USP <1857>, the new EP standard is extended to encompass HPLC UV detectors and PAT (process analytical technology) applications. This is a considerable divergence – USP <857> specifi cally excludes HPLC detectors from its scope whereas EP 2.2.25 will be mandatory for HPLC detectors. The following notes relate to spectrophotometers: HPLC is covered separately.
Another major difference is in the approach to the test materials to be used for qualifi cation. The USP states: ‘Whenever possible, certifi ed reference materials (CRMs) are to be used in preference to laboratory-prepared solutions’ and now lists a greater variety of commercially available CRMs to make it easier to establish ‘fi tness for purpose’ over a range of operating parameters. The EP, conversely, gives details for the preparation of relatively few test solutions, one of which (caffeine) is not available commercially as a CRM. Fortunately, however, it still allows the use of Certifi ed Reference Materials, so compliance can still be achieved without the need to prepare solutions in the laboratory.
Will I need new reference materials?
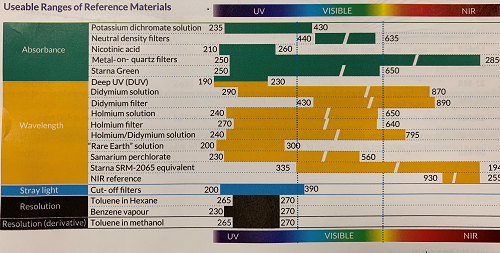
Most laboratories working in a regulated environment will already have a selection of references for instrument qualification. To determine if any additional references are needed to meet 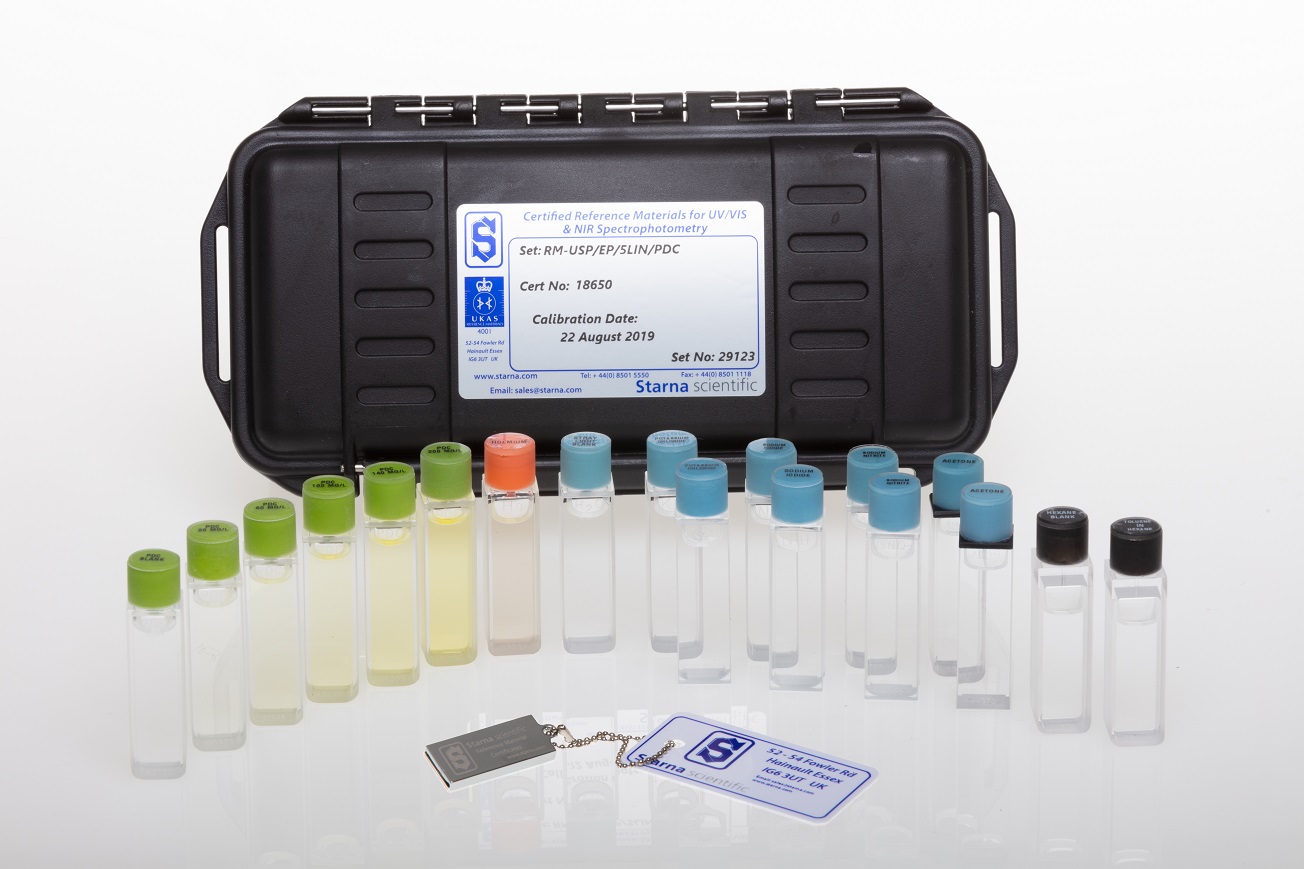 the new regulations the wavelength and absorbance values expected in the proposed analyses should be checked to see if they are encompassed by the available references. If not, additional references will be required. For example, holmium oxide solution is the most widely used wavelength reference, with 14 peaks covering wavelengths from 240 nm to 650 nm. Provided the wavelengths to be used for analysis lie within these limits, no additional wavelength references should be required. For wavelengths below 240 nm, however, both standards now recommend cerium oxide solution, covering 200 nm to 270 nm. For even lower wavelengths, a ‘Deep UV’ CRM is available from Starna Scientific (Hainault UK), with certified wavelength and absorbance values down to 191 nm. When including analysis above 650 nm, didymium oxide is frequently used.
the new regulations the wavelength and absorbance values expected in the proposed analyses should be checked to see if they are encompassed by the available references. If not, additional references will be required. For example, holmium oxide solution is the most widely used wavelength reference, with 14 peaks covering wavelengths from 240 nm to 650 nm. Provided the wavelengths to be used for analysis lie within these limits, no additional wavelength references should be required. For wavelengths below 240 nm, however, both standards now recommend cerium oxide solution, covering 200 nm to 270 nm. For even lower wavelengths, a ‘Deep UV’ CRM is available from Starna Scientific (Hainault UK), with certified wavelength and absorbance values down to 191 nm. When including analysis above 650 nm, didymium oxide is frequently used.
For absorbance qualification, the new linearity specifications mean that more than one reference will almost certainly be required: the USP recommends that linearity is controlled at a minimum of three absorbance levels over the expected range. Potassium dichromate solution has been used for many years and covers wavelengths from 235 to 350 nm. For lower wavelengths, nicotinic acid is now recommended, covering 210-270 nm. Both are commercially available as CRMs, at a range of concentrations with absorbance values up to 3A and can be purchased in convenient ‘linearity sets’. For the visible region, the USP now lists metal-on-quartz filters; while not compatible with all instruments, these filters can be used over a wide wavelength range (250 to 850 nm). For the visible region, wellestablished neutral density (grey glass) filters are available.
Stray light qualification: whereas EP 2.2.25 formerly named just one stray light reference, potassium chloride solution, both standards now list several references covering wavelengths from 190 nm to 400 nm. Note that USP <857> now permits the use of the traditional “specified wavelength” method as well as the ‘filter ratio’ or Mielenz method cited in the 2015 edition.
Both standards recommend the well-established toluene-in-hexane solution for resolution (bandwidth) qualification.
Other than the references named in the standards, a wide range of CRMs for spectrophotometry is available (see table below):







 Savillex has launched a new generation C-Flow s-type series of PFA Nebulizers. These are specially designed for ICP-MS analysis of semiconductors and similar demanding low volume ICP-MS analyses. The s-type uniquely combines the sensitivity and excellent washout of a Nebulizer with integrated suction line with the versatility and ease of a demountable Nebulizer.
Savillex has launched a new generation C-Flow s-type series of PFA Nebulizers. These are specially designed for ICP-MS analysis of semiconductors and similar demanding low volume ICP-MS analyses. The s-type uniquely combines the sensitivity and excellent washout of a Nebulizer with integrated suction line with the versatility and ease of a demountable Nebulizer.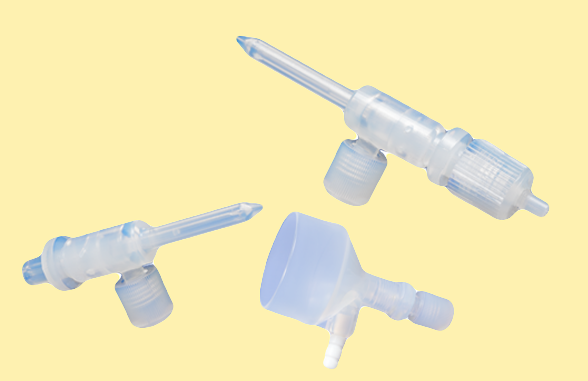






 the new regulations the wavelength and absorbance values expected in the proposed analyses should be checked to see if they are encompassed by the available references. If not, additional references will be required. For example, holmium oxide solution is the most widely used wavelength reference, with 14 peaks covering wavelengths from 240 nm to 650 nm. Provided the wavelengths to be used for analysis lie within these limits, no additional wavelength references should be required. For wavelengths below 240 nm, however, both standards now recommend cerium oxide solution, covering 200 nm to 270 nm. For even lower wavelengths, a ‘Deep UV’ CRM is available from
the new regulations the wavelength and absorbance values expected in the proposed analyses should be checked to see if they are encompassed by the available references. If not, additional references will be required. For example, holmium oxide solution is the most widely used wavelength reference, with 14 peaks covering wavelengths from 240 nm to 650 nm. Provided the wavelengths to be used for analysis lie within these limits, no additional wavelength references should be required. For wavelengths below 240 nm, however, both standards now recommend cerium oxide solution, covering 200 nm to 270 nm. For even lower wavelengths, a ‘Deep UV’ CRM is available from