Say goodbye to DIY with ready-to-use reagents for discrete analyzers! Until now, pre-mixed reagents have not been offered in a sustainable, reliable way. Through extensive research and development, Inorganic Ventures has created the perfect alternative to in-house reagents. These products require no preparation time, improving your efficiency and minimizing errors.
In addition to saving time, these reagents offer method flexibility, batch-to-batch consistency and comprehensive stability data.
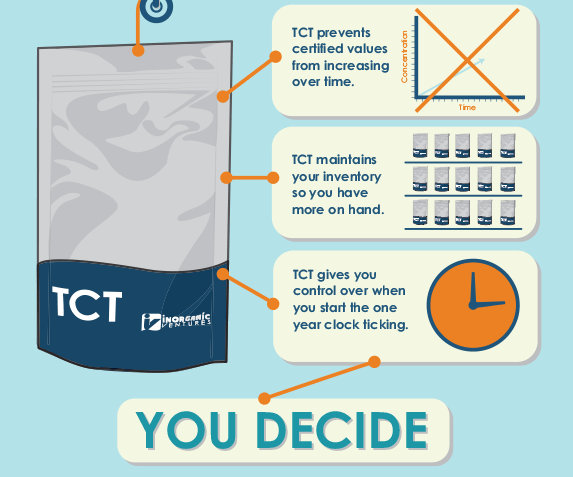
As always, these products are packaged in Inorganic Ventures’ Transpiration Control Technology (TCT) for protection from light and you can have them in your home within 2 weeks.
EPA or standard methods for which reagents are available: (*)
- Chloride (Cl-) Method – Standard Method 4500-Cl-E, EPA Method 325.2
- Phosphate (PO4-3) Method – Standard Method 4500-PO4-3E
- Sulfate (SO4-2) Method – Standard Method 4500-SO4-2E, EPA Method 375.4 – Sulfate, Turbidimetric, ASTM D516-11 – Standard Test Method for Sulfate Ion in Water, ISO 15923-1 – Water Quality- Determination of Selected Parameters by Discrete Analysis Systems – Part 1: Ammonium, Nitrate, Nitrite, Chloride, Orthophosphate, Sulfate and Silicate with Photometric Detection
- Ammonia (NH3) Method – ISO 15923-1, ISO 7150/1, EPA Method 350.1, Standard Methods 4500-NH3 F
(*) Please keep an eye on our website. This year the suite of reagents will be further expanded! Likely to be: Ammonia. Alkalinity, Hardness, Chromium and more. Let us know which ones you are looking for!
Shelf life
Because this is a new product, the manufacturer Inorganic Ventures is conducting rolling stability studies. The shelf life is now at 9 months because the study is now 9 months in progress. During the study, every three months the stability is extended by three months until the maximum shelf life is reached, which is not clear at this time.
Not sure if these reagents are right for your laboratory? Below we have listed some of the most frequently asked questions with our answer.

Win back time and increase your lab’s efficiency with ready-to-use reagents! FAQs
1. What instruments are the discrete analytical reagents designed for?
Our discrete reagents can be used on the following instruments:
2. I am using a segmented flow analyzer. Are your discrete analyzer reagents compatible with this instrument?
The reagents for discrete analyzers can work on your segmented flow analyzer. Please refer to the Method Chemistry Sheets (links above) for each product for detailed product composition. Please contact us directly with any questions.
3. Does shipping to or through locations with hot temperatures cause stability issues with the reagents?
Our stability studies have shown that some reagents can be sensitive to heat. All of our products undergo shipping stability to ensure that temporary exposure to heat does not affect product performance. At this time, we recommend refrigerated storage of discrete analytical reagents until our stability studies are completed and storage recommendations on the product coAs are reviewed.
4. Transpiration Control Technology (TCT) extends the shelf life of Inorganic Ventures’ standards by up to five years. Do the Discrete Analyzer Reagents have the same 5-year shelf life?
Unfortunately no. Our standards have a 5-year chemical stability and the TCT pouch ensures that the concentration does not change due to perspiration. The discrete analyzer reagents are still being tested for chemical stability and we currently use the TCT pouch for protection from light. Currently, all of our discrete reagents have a shelf life of 9 months and we will adjust the shelf life every 3 months for the duration of stability testing.
5. The 500 ml volume is not suitable for my testing needs. Does Inorganic Ventures offer other bottle sizes?
Whether you need a custom volume size or a unique formulation, we are happy to work with you to meet your needs. To get started, contact us!
6. I’m not sure if I’m really saving time and money by switching from reagents made in my lab to off-the-shelf reagents from Inorganic Ventures. How do I know for sure?
Schedule an appointment with our Product Manager Jeroen van Boxtel and we’ll walk you through your individual time and money savings! We have a savings calculator ready and you can take the findings to your team.
7. My standard operating procedure (SOP) is very complex. I’m not sure if I can easily add new reagents to the workflow. Can you help?
No problem! Inorganic Ventures’ technicians specialize in SOP development and implementation. Make an appointment through us with one of these chemists for technical support to discuss your specific SOP requirements.
8. I am following alternative methods on my discrete analyzer, are there other methods available?
We are launching more reagents for discrete analyzers in the coming months! The following – Ammonia. Alkalinity, Hardness, Chromium and more to follow. Let us know which ones you are looking for!

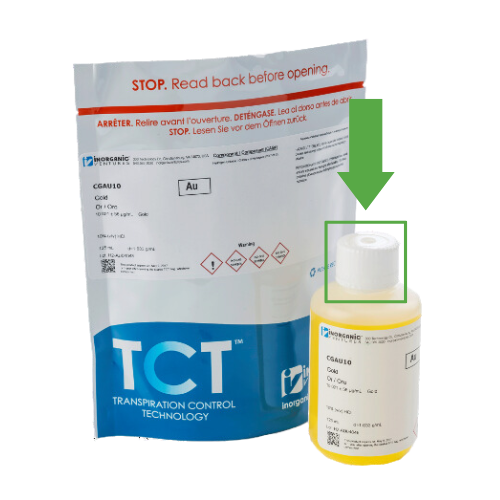















 Fortunately, there is an alternative, namely
Fortunately, there is an alternative, namely 

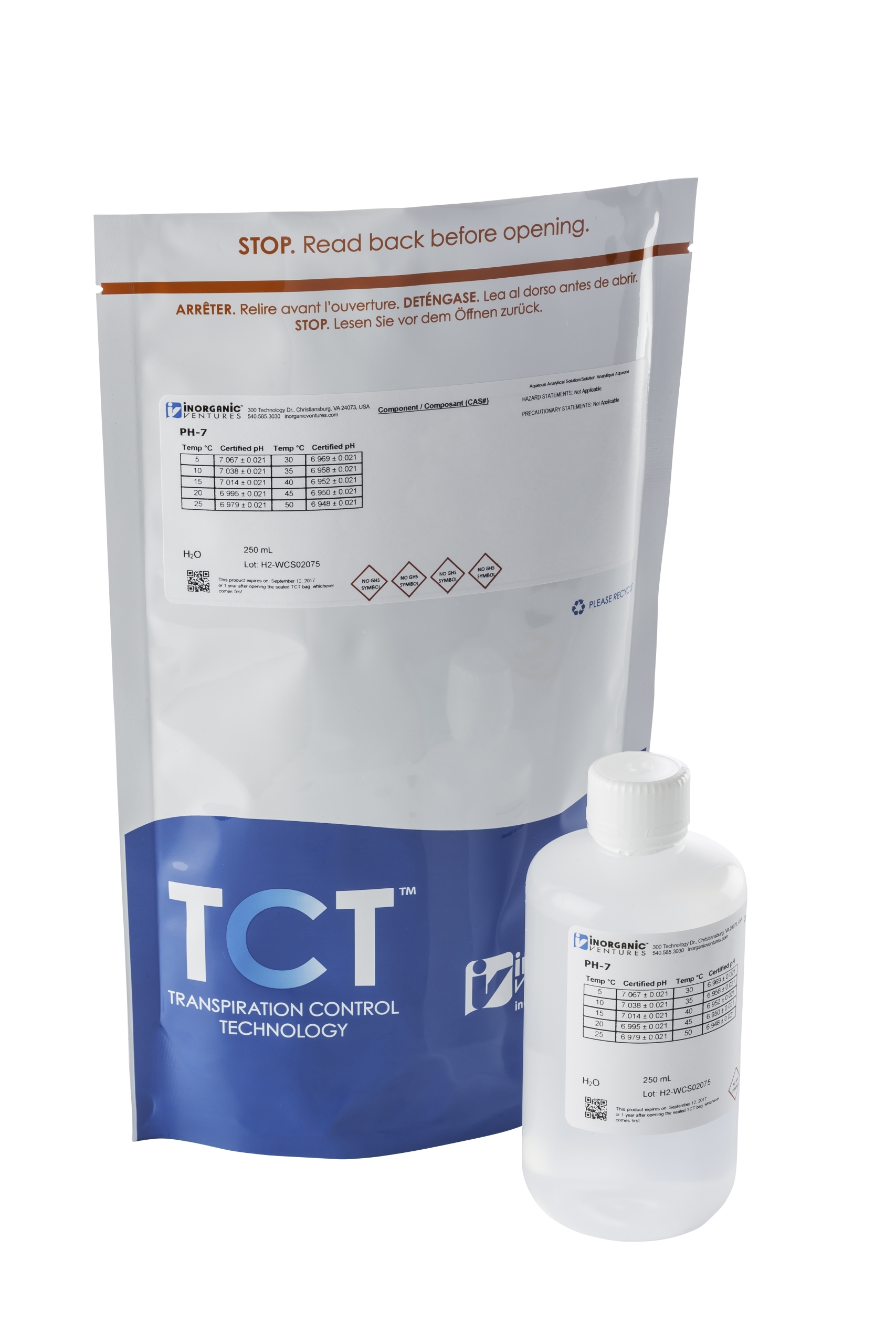 Verpakt in Transpiration Control Technologie (TCT), die de wetenschappelijke integriteit garandeert tot 5 jaar vanaf de datum van productie.
Verpakt in Transpiration Control Technologie (TCT), die de wetenschappelijke integriteit garandeert tot 5 jaar vanaf de datum van productie.